Ethanoic Acid and Sodium Hydroxide
The chemical equation of the reaction is as follows. But ethnaote ion is not stable in the water and hydrolysis the water and release hydroxyl ions.
When same amount mol of sodium hydroxide and ethanoic acid are mixed mixture contains sodium ethanoate.
. Page 6 of 11. The unbalanced chemical equation that describes this neutralization. Solution a When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed.
Ethanoic acid Acetic acid CH³COOH is an acid while Sodium hydroxide NaOH is a base. The salt is sodium acetateAcetic acid reacts with sodium hydroxide a base according to the. The IUPAC name of.
Graph the pH versus NaOH added. Slowly add a 1M solution of. CH 3COOHNaOH sodium ethanoateCH.
Ethanoic acid and NaOH Sodium ethanoate and water are given as products by the reaction of ethanoic acid and aqueous NaOH. Sodium ethanoate dissociate completely in the water to ethnaote ion and sodium ion. Identify solution should be used as a titrant.
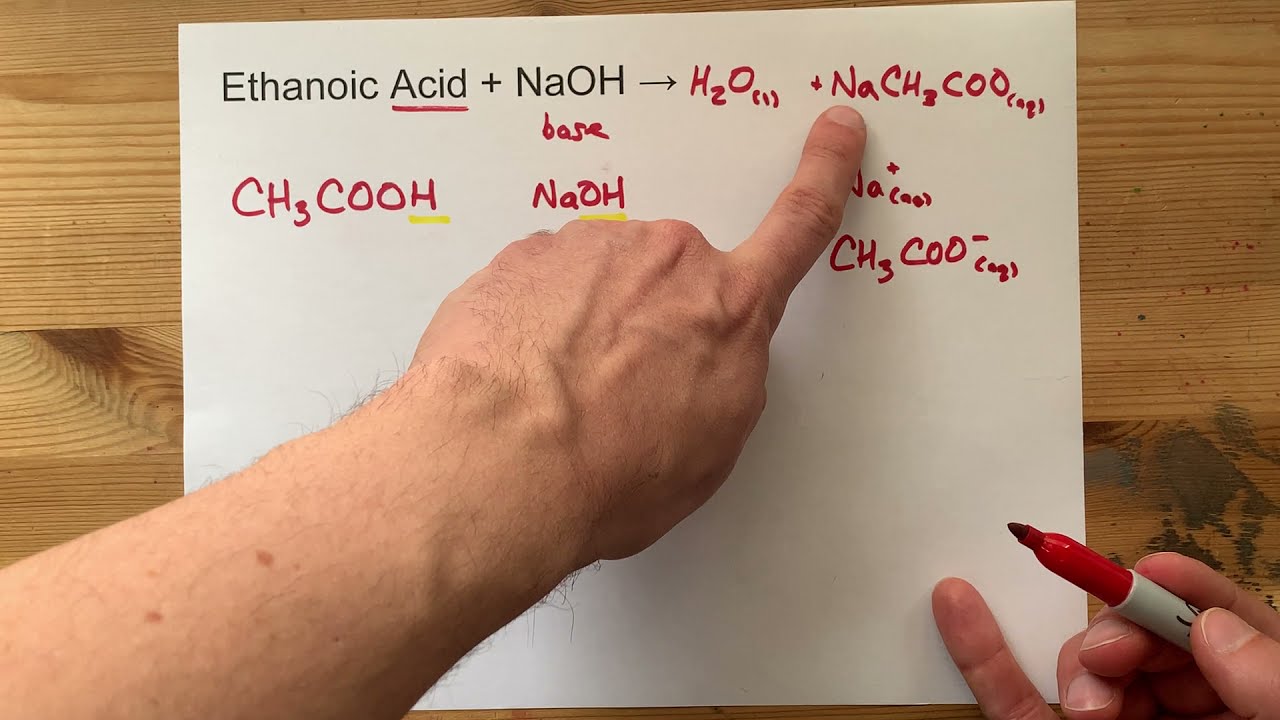
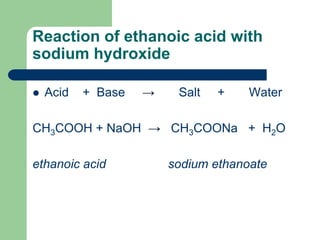
How do you neutralize acetic acid and sodium hydroxide. Reaction of ethanoic acid CH 3 COOH with sodium Na. Acetic acid CH3COOH will react with sodium hydroxide NaOH to produce sodium acetate CH3COONa and water.
The reaction of Ethanoic acid with a base Ethanoic acid undergoes a neutralisation reaction with a strong base like sodium hydroxide to form salt and water. What happens when sodium hydroxide is added to ethanoic acid. The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid.
Strong bases are considered. Slowly add acetic acid to a container of cold water to form a 110 dilution of acid to water. Ethanoic acid is a weak organic acid that combines with sodium metal to generate sodium ethanoate CH 3 COO-Na also known.
Titration of adding sodium hydroxide to ethanoic acid Aqueous ethanoic acid shows a pH value below. Ethanoic acid is commonly known as acetic acid. Sodium ethanoate is a salt and soluble in water.
The balanced chemical reaction between ethanoic acid and sodium metal is shown below. What salt is made when sodium hydroxide and acetic acid react together. So their reaction gives us sodium salt of Ethanoic acid water as follows.
The reaction of ethanoic acid with sodium metal results in the formation of sodium ethanoate or commonly known as sodium acetate and hydrogen gas is liberated. Draw and fully label a titration curve from the data collected from the acetic ethanoic acid and sodium hydroxide titration. 25 mL of a 0095M of acetic acid was diluted to 100mL with deionized water and titrated with 0101M of NaOH.
For the titration of ethanoic acid CH3COOH with sodium hydroxide NaOH a. Suggest the pH at the equivalence point either as. The equation for the reaction occurring is CH3COOH aq NaOH aq -.

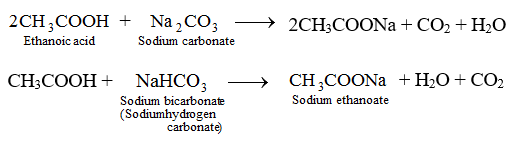
Write Chemical Equations For What Happens When Sodium Metal Is Added To Ethanoic Acid Solid Sodium Carbonate Is Added To Ethanoic Acid Ethanoic Acid Reacts With A Dilute Solution Of

Physical Amd Chemical Properties Of Ethanoic Aacid Chemistry Knowledgeuniverseonline Com

No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment